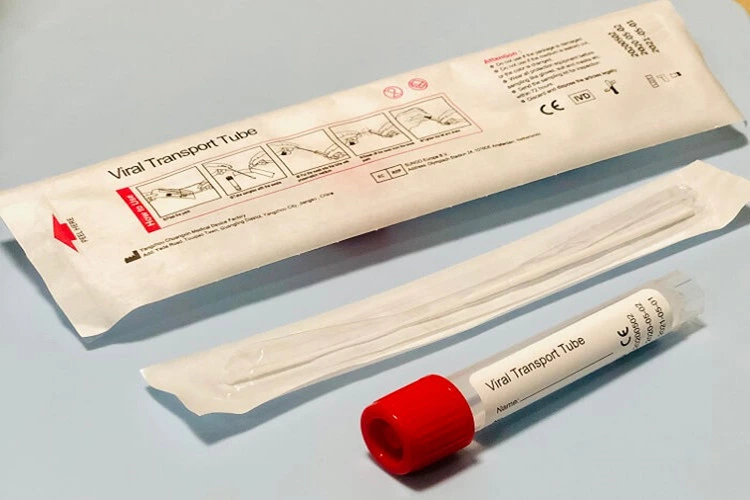
Inactivated Viral Transport Medium
Viral transport Medium Reference No. Specification Swab type Transport medium type VTM-1 10ml tube ,3ml transport medium Swab Rayon tip Non-inactivated VTM-2-1 10ml tube ,3ml trans
Viral transport Medium
| Reference No. | Specification | Swab type | Transport medium type | |||
| VTM-1 | 10ml tube ,3ml transport medium | Swab Rayon tip | Non-inactivated | |||
| VTM-2-1 | 10ml tube ,3ml transport medium | Oral swab +Nasal swab Flocked tip | Non-inactivated | |||
| VTM-2 | 10ml tube ,3ml transport medium | Oral swab Flocked tip | Non-inactivated | |||
| VTM-3 | 10ml tube ,3ml transport medium | Nasal swab Flocked tip | Non-inactivated | |||
| VTM-3-1 | 10ml tube ,3ml transport medium | Oral swab +Nasal swab Flocked tip | Inactivated | |||
| VTM-4 | 10ml tube ,3ml transport medium | Swab Rayon tip | Inactivated | |||
| VTM-5 | 10ml tube ,3ml transport medium | Oral swab Flocked tip | Inactivated | |||
| VTM-6 | 10ml tube ,3ml transport medium | Nasal swab Flocked tip | Inactivated | |||
| VTM-7 | 6ml tube, 2ml transport medium | Oral swab Flocked tip | Inactivated | |||
| VTM-8 | 6ml tube, 2ml transport medium | Nasal swab Flocked tip | Inactivated | |||
| VTM-9 | 6ml tube, 2ml transport medium | Nasal swab +oral swab flocked tip | Inactivated | |||
Instructions for Virus Transport tube
Intended Use
- Collection, preservation and transportation of human nasopharyngeal or oropharyngeal virus samples.
Description
- The system is mainly composed of storage tube, preservation medium, and swabs. The medium is based on Hank’s balanced salt solution. The system has a stable osmotic pressure, which provides a appropriate transport and storage environment for swab samples. It can maintain virus samples under prescribed conditions for routine nucleic acid extraction, genetic testing and PCR testing, etc.
Specification
- Medium: Non-inactivated or Inactivated
- Package: 30 pcs/box, 50pcs/box, 60pcs/box
Difference in Medium
| Non -Inactivate Medium | Inactivate Medium | |
|---|---|---|
| Medium Color | Pink color | Little yellow |
| Formula | Not include lysis solution | Include lysis solution |
| Function | maintain activity and integrity of the pathogen | It can instantly lyse the pathogen and release nucleic acid, the protective agent can prevent nucleic acid from being degraded. |
| Sample preservation | 2-8℃, 24hours | Room temperature, 30days |
| advantage | It can be used not only for the extraction of nucleic acid, but also for the culture and isolation of pathogens. | It is suitable for follow-up detection after nucleic acid extraction |
Main components
| VTM kits Include | Description |
|---|---|
| Flocked Swab | 1pcs Nasopharyngeal swab or(and) 1pcs oropharyngeal swab |
| Tube | Tube size: 16mmx 100mm or 13mm× 84mm |
| Medium(Non-inactivated) | Appearance: Purple red or clear pink liquid Volume: 3 ml or customized volume |
| Medium(inactivated) | Appearance: Purple red or clear pink liquid Volume: 3 ml or customized volume |
Sampling procedures
- Peel the pack and use a swab for sampling in the nose or throat according to different sampling requirements.
- Nasopharyngeal swab: Gently insert the swab head into the nasal condyle of the nasal passage, stay for a while and then slowly screw the swab to turn it out.
- Oropharyngeal swab: Gently wipe the bilateral pharyngeal tonsils and posterior pharyngeal wall with the swab.
- Insert swab into the storage tube and immerse the swab head into the medium
- Break shaft gently from the breakpoint, discard the tail and tighten the tube cap.
- Mark the relevant sample information on the label of the storage tube.
- Freshly collected clinical specimens should be delivered to the laboratory within 72 hours at 2℃- 8℃.
- Nucleic acid extractiondetection
- For the Non-inactivated type, before extracting and detecting nucleic acids, the virus inactivation and lysis must be performed with referring to the relevant standard steps of nucleic acid extraction or the instruction manual of the extraction reagent to perform.
- For the inactivated type, normal virus inactivation and lysis can still be performed as required. And as the inactivated samples contain free viral nucleic acid, so the nucleic acid extraction and detection operations can be directly done with referring to relevant standard steps of nucleic acid extraction or the instruction manual of
- Suggested Temperature: 4°C -25°C.
- Period of validity: 12 months.
- Please refer to the outer case for the manufacture date and expiration date.
Transportation
- Samples collected with nasopharyngeal or oropharyngeal swabs should be transported at 2°C – 8°C and submitted for test as soon as possible.
- After sampling, transport and storage time for specimen should be no longer than 72h.
Sample deterioration
- If the collected sampling medium turns yellow or turbid, discard it and re-sample with a new piece of kit.
Limitations
- Contamination during sampling process will affect the final results.
- Sample storage at inappropriate temperature will affect the final results.
Attention
- This product is for in vitro diagnostic use only.
- Do not use if it is out of date or damaged.
- Do not use if the medium turns yellow or leaked out.
- This product is intended for using by trained laboratory personnel only.
- Gloves, masks and gowns should be worn for personal security while using the product.
- All biologically hazardous specimens and devices should be handled in accordance with relevant regulations and procedures, and discarded in designated medical waste collection containers as required.
- This product is only used for storage after sample collection and in vitro transportation. It is only for one-time use and does not have a cultivation function.